|
|||||
|
The background, the facts, figures, politics and analysis. The Science How is oil created?
Oil is a finite resource, so the question really should be how WAS oil created. It was made at one unique period in the earth's geological history and as readers will, no doubt, be aware it is not universally distributed - it occurs in well defined geological situations which are found throughout parts of the middle east, North America, Mexico, Venezuela, Nigeria, the North Sea, the Caspian sea, central Asia and Mediterranean Africa.
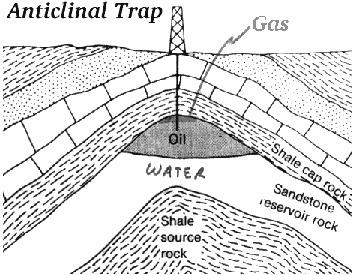
Figure: Rock formations which lead to entrapment of oil.
How is oil extracted? Geologists have a pretty complete picture of the earth's surface and can identify the geological locations which have the right circumstances for holding oil deposits. It doesn't mean to say that the rock structure will bear any oil at all. The only sure way of proving the existence of oil is to drill for it. As mentioned above, oil is found trapped under non-porous rock and consequently the rock has be to drilled into in order to extract the oil. The drill bit is made of steel with industrial diamonds on the cutting edges. The drill bit is connected to the end of sections of spinning pipe which are manually put together by workers on the oil rig (sometimes called a platform). The drill creates a hole which is called a well. An oil field may cover several square miles. There may be many wells drilled into the rock at various locations within the oil field. In the North Sea most oil wells are between 900 and 5,000 metres deep, but a drill can go down as far as 8,000 metres (5 miles)The wells also travel horizontally through the reservoir. Some parts of the North Sea are 200m deep (656ft) so the drill thread has to be carefully lowered to the sea bed before it can start drilling through the cap rock. Some oil reservoirs need to be pumped. In this case, there are small pumps inside the well pipes. Also, water is pumped into the well to force the crude oil mixture out. This water is pumped down injector wells while the oil is pumped out of production wells. On the Captain platform, owned by BP, just off the north coast of Scotland in the Moray Firth there are 27 such wells operated from the single platform. Five of these act as injector wells which conduct water which is pumped into them. The 22 other wells are the receiving wells up which the oil is forced by the pressure of the pumped water. The top end of the well is called the well head . This is where the oil is removed from the well for onward discharge into pipeline or tanker ship. North Seas oil platform are huge structures. Some have concrete which sit on the sea bed itself. Others have metal legs which have been jacked up, while a few are floating structures. The North Sea has over fifty seperate oil fields. But all of them reached their peak output some years ago. That means they are now in decline. No new untapped fields have been found. The UK now has to import crude, mainly from the Middle East, to meet current consumption demands. Table: North Sea Oil Field Data
Once extracted the crude has to be purified(refined) before it can be put to commercial use. Such uses include petrol (which the Americans call "gas") in vehicles, diesel for use in other vehicles, aviation fuel, feedstock for the chemical industry and bitumen for asphalt manufacture. Table: Destination of refined oil products
What happens in a refinery There are 8 oil refineries in the UK; situated at Grangemouth, Middlesborough, Stanlow, Killingholme, Coryton, Milford Haven, Llandarcy, and Fawley. All of these are coastal sites and some have chemical plants within their complexes. The crude oil brought to the refineries by tanker or pipeline is a mixture of compounds - mainly hydrocarbons. (A hydrocarbon is a chemical compund made up of just carbon and hydrogen, e.g. methane, butane, propane, octane.) Fractional distillation splits the crude oil into simpler mixtures called fractions. The different fractions are taken away from the still at different elevations by pipes for further treatment. The crude oil is heated in a furnace to about 370°C and is pumped into the bottom of a distillation tower. Most of the hydrocarbons are gaseous, though the very thick ones are still a liquid even at this temperature. Two stills are commonly used; first the atmospheric still with the residue from this being passed to the vacuum still where it is split into more fractions. The tower is like a giant heat exchanger - it removes heat from the gases as they rise up it. The temperature falls to 20°C by the time the vapours reach the top. The vapours condense as they rise up the tower. The heavier ones (with higher boiling points) condense first. The thinner, more mobile ones get further up the tower before they condense. And the gases pass out of the top. The temperature gradually decreases further up the column. Different groups of hydrocarbons condense at different heights. The heaviest at the bottom, the lightest at the top. Cracking The crude oil product which has the biggest demand is petrol, which is made from the naphtha fraction. This fraction contains molecules with 5 to 10 carbons in their chains. As such, it is one of the lighter fractions. Although about 20% of a barrel of crude oil eventually goes to petrol, there is nowhere near this amount of naphtha in the raw crude. So, the demand is much greater than what is available. However, there is a surplus of heavier fractions like heavy gas oil, which has very few direct uses. Its molecules have more than 20 carbons in their chains. To meet demand for petrol, chemists have devised a solution, which is to break up these long chain molecules into the shorter molecules that are useful in, for example, petrol. This process is called "cracking". The cracking reaction can be induced using high temperatures (about 500 C) in the presence of steam or a zeolite catalyst. The steam cracker reacts steam with heavy gas oil. The heavy gas oil is called the feedstock - i.e. it is what is fed into the cracker. The long chain hydrocarbons are split into much shorter chains with 1 to 8 carbon atoms. The main product is ethane (with 2 carbons). The C5 to C8 hydrocarbons go into the petrol blend. The products pass out of the top of the reactor vessel to a fractionating column to be separated. About 20% of the product goes into petrol. The rest are lighter hydrocarbons which pass into the refinery processes. The catalyst flows back into the regenerator where it is reheated to burn of the carbon ready for another round of catalysing. An excellent animation of the catalytic cracking process can be found here. Table: Fractions of crude oil
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
All material may be freely reproduced and distributed in any medium for non-profit use.
Please acknowledge source of the material as the
British National Party , PO Box 287, Waltham Cross, Herts,
EN8 8ZU. Telephone: 0870 7576 267
Please mention this website when copying material.